Modalities
We offer a broad range of modalities aligned with therapeutic targets and research objectives.
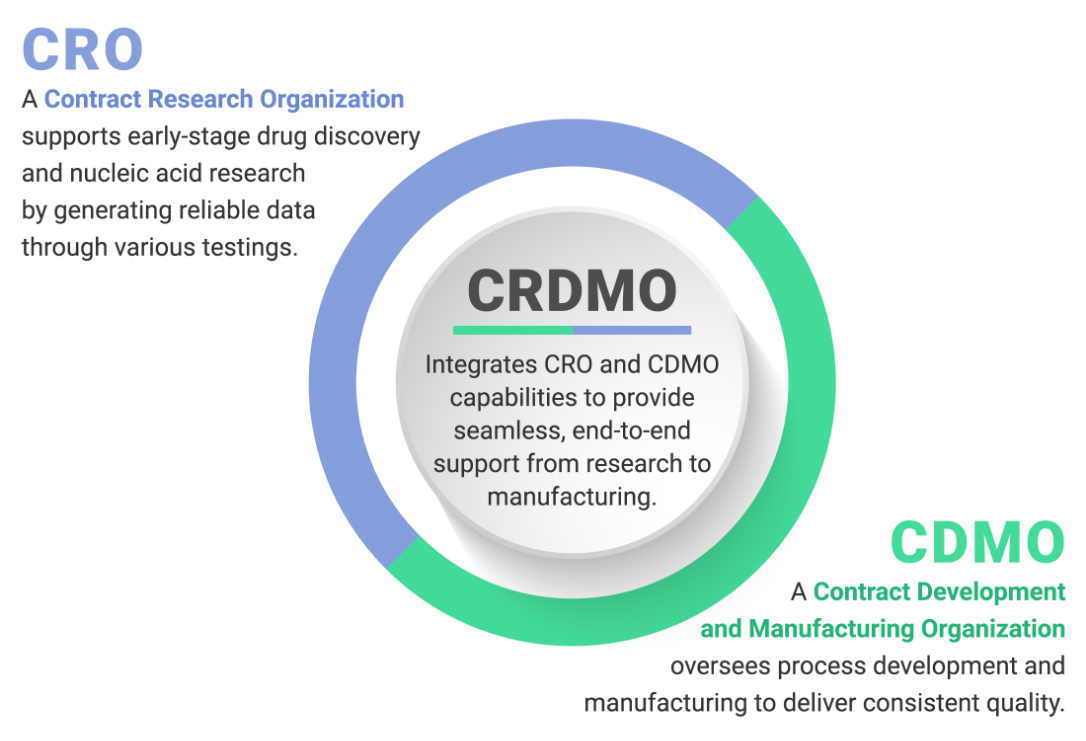
AXELPHEX™ is a jointly operated CRDMO platform by Mitsubishi Gas Chemical Company (MGC) and Hokkaido System Science (HSS) for oligonucleotide APIs.
Through an integrated framework that ensures stepwise optimization and seamless transition from research to GMP manufacturing, AXELPHEX™ combines efficiency with robust quality assurance. This platform provides flexible engagement from ad-hoc assignment on any process to fully integrated end‑to‑end project.
Target mRNA structures are analyzed and predicted to identify regions suitable as therapeutic targets.
Gapmer-type ASOs are then designed and optimized to support effective candidate selection.
From drug discovery to preclinical evaluation, we provide customized synthesis services optimized for your research purpose, adjusting modifications, purity and scale for both in vitro and in vivo use.
We also support custom modifications beyond our standard offerings as well as custom synthesis using client-supplied amidites. Please feel free to contact us.
We offer a broad range of modalities aligned with therapeutic targets and research objectives.
Various modifications are available to support enhanced stability, specificity and pharmacokinetic (PK) properties.
We support conjugation strategies to enhance organ and tissue targeting and cellular uptake efficiency.
Supply of small quantities with multiple sequences—ideal for screening.
Available in sodium salt form and supplied under endotoxin-controlled conditions.
We offer acyclic artificial nucleic acids developed by Professor Hiroyuki Asanuma’s laboratory at Nagoya University.
Leveraging their characteristics—stable duplexes with canonical DNA or RNA, strong resistance to nuclease degradation and lower cytotoxicity—SNA and iL-aTNA can be utilized for design, synthesis and evaluation tailored to your objectives.
Available from January 2026
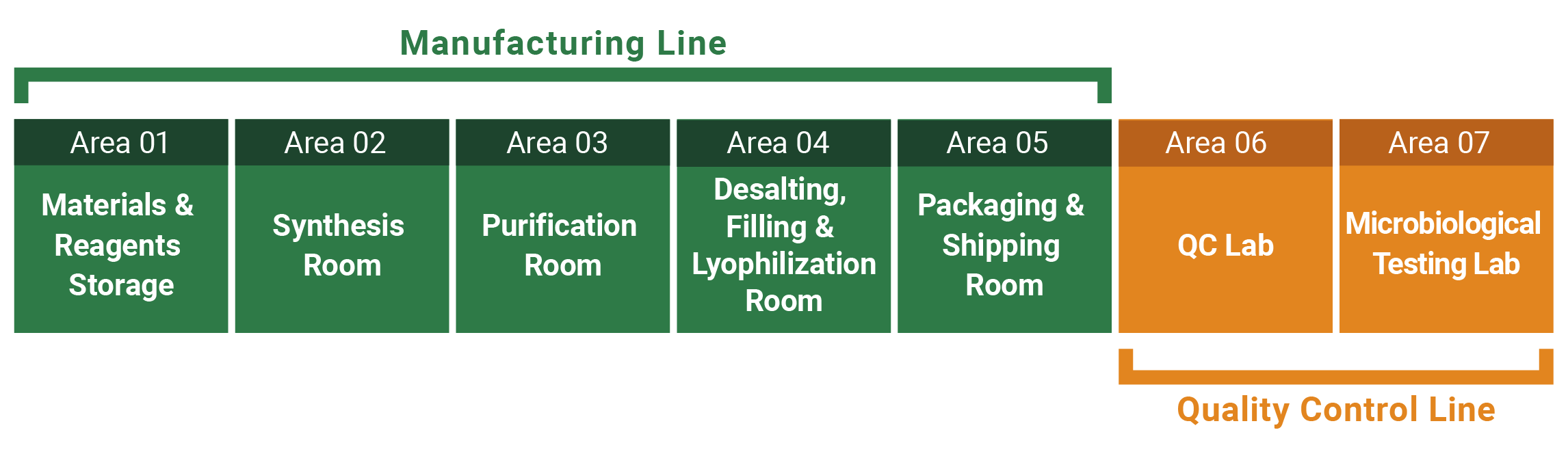
Manufacturing is conducted under a management system compliant with GMP and PIC/S.
Manufacturing methods, scales and analytical conditions are flexibly designed according to project objectives and development stages.
Available from January 2026
We provide contract analytical services covering a wide range of quality tests required for nucleic acid therapeutics.
Analytical method development and synthesis of impurity reference materials (e.g., N-1/N+1 and depurinated species) are also available.
| No | Test Item | Equipment |
|---|---|---|
| 1 | Appearance | — |
| 2 | Purity (Assay) | HPLC |
| 3 | pH | pH meter |
| 4 | Identification Test (Molecular Weight) | Q-TOF LC/MS |
| 5 | Identification Test (Sequence) | Q-TOF LC/MS |
| 6 | Purity and Impurity Profile | Q-TOF LC/MS, HPLC |
| 7 | Tm Value (Duplex) | UV-Vis Spectrophotometer with Tm Analysis System |
| 8 | Water Content | Karl Fischer Moisture Titrator |
| 9 | Sodium Content | ICP-MS |
| 10 | Elemental Impurities | ICP-MS |
| 11 | Residual Solvents | GC-MS |
| 12 | Endotoxin | Toxinometer |
| 13 | Total Microbial Count | Membrane Filtration System |
We are able to offer in vitro screening, pharmacological efficacy testing and safety evaluation.
Support is available throughout from assay design to validation tailored to your research objectives.
RNA‑seq and microarray are available for comprehensive transcriptomic analysis of gene expression changes after oligonucleotide administration.
Results assist to understand off‑target effects as well as mechanism of action.
We are able to offer in vivo pharmacological, pharmacokinetic (PK) and toxicity studies.
Testing is conducted in accordance with ICH guidelines for preclinical and clinical studies.
We look forward to hearing about your research projects including development stage, objectives, planned production scale as well as analytical testing.
Based on your requirements, we will propose the most suitable plan.
Contact